12 C2h4 Facts To Boost Chemistry Skills

Ethylene, commonly known by its chemical formula C2H4, is a fundamental compound in organic chemistry, playing a crucial role in various industrial and biological processes. As the simplest alkene, ethylene's unique properties and reactivity make it an essential component in the production of numerous chemicals, materials, and fuels. Understanding C2H4 is vital for chemists, researchers, and students seeking to advance their knowledge in chemistry, particularly in areas such as petrochemistry, polymer science, and agricultural chemistry. This article delves into 12 key facts about C2H4, aiming to enhance readers' comprehension of its characteristics, applications, and significance in the chemical industry.
Key Points
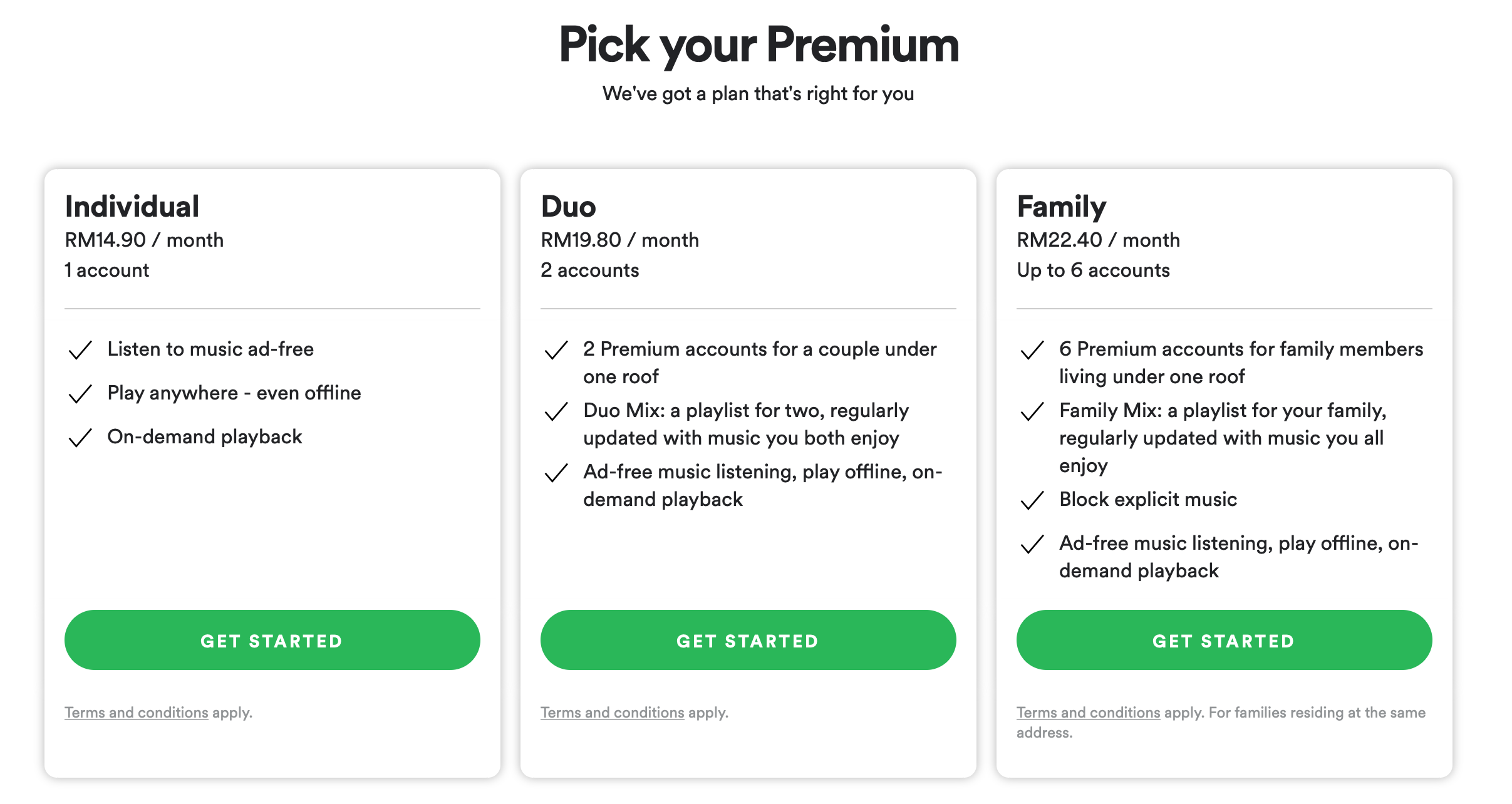
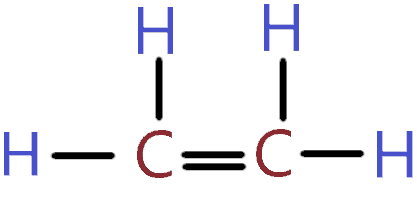
- Ethylene (C2H4) is the simplest alkene, with a molecular structure consisting of two carbon atoms bonded to each other through a double bond, and each carbon is also bonded to two hydrogen atoms.
- C2H4 is highly reactive due to its double bond, participating in a wide range of chemical reactions, including addition reactions, polymerization, and substitution reactions.
- Ethylene is produced industrially through the steam cracking of hydrocarbons, such as ethane and propane, which is a critical process in the petrochemical industry.
- The compound is a key building block in the synthesis of various polymers, notably polyethylene, which is one of the most widely used plastics globally.
- C2H4 plays a significant role in plant biology, acting as a hormone that regulates fruit ripening, senescence, and stress responses in plants.
- Ethylene's physical properties include being a colorless gas at room temperature, with a characteristic sweet odor and a molecular weight of approximately 28.05 g/mol.
- In terms of safety, C2H4 is considered a hazardous substance due to its flammability and potential to form explosive mixtures with air.
- The compound has various applications beyond its use in polymer production, including as a refrigerant, an anesthetic, and in the production of ethylene oxide, which is used in the manufacture of antifreeze and other chemicals.
- Ethylene is also a critical component in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC), another widely used plastic.
- Research into ethylene's biological functions continues to uncover its importance in human health and disease, including its potential role in inflammation and cancer.
- Given its significance, the production and use of C2H4 are subject to environmental and safety regulations, aiming to minimize its impact on human health and the environment.
- Understanding the chemistry of ethylene is essential for the development of new technologies and materials, as well as for addressing the challenges associated with its production and use.
Chemical Structure and Properties of C2H4
The chemical structure of ethylene is characterized by a planar, unsaturated hydrocarbon with a double bond between the two carbon atoms. This double bond is responsible for the high reactivity of C2H4, as it can easily participate in addition reactions. Ethylene’s molecular formula, C2H4, indicates that each carbon atom is bonded to two hydrogen atoms, in addition to the carbon-carbon double bond. This configuration results in a molecule with a relatively low molecular weight and high volatility. In terms of physical properties, ethylene is a colorless gas with a sweet odor, which is noticeable at concentrations above 250 ppm. Its boiling point is approximately -103.7°C, and it has a critical temperature of 9.7°C, making it a gas at room temperature under standard pressure conditions.
Industrial Production of Ethylene
The industrial production of ethylene primarily involves the steam cracking of hydrocarbons. This process entails the thermal decomposition of ethane, propane, or naphtha in the presence of steam, resulting in the formation of ethylene and other olefins. The choice of feedstock depends on various factors, including availability, cost, and the desired product mix. The steam cracking process is carried out at high temperatures, typically in the range of 750°C to 900°C, and under moderate pressure. The resulting ethylene is then purified through a series of distillation and absorption steps to produce high-purity ethylene, which is essential for many downstream applications.
| Property | Value |
|---|---|
| Molecular Formula | C2H4 |
| Molecular Weight | 28.05 g/mol |
| Boiling Point | -103.7°C |
| Critical Temperature | 9.7°C |
| Odor | Sweet |

Applications of Ethylene in Polymer Production
One of the most significant applications of ethylene is in the production of polyethylene, which is achieved through the polymerization of ethylene monomers. Polyethylene is a versatile polymer used in a wide range of applications, from packaging materials and household goods to medical devices and automotive components. The polymerization process can be tailored to produce different types of polyethylene, including low-density polyethylene (LDPE), high-density polyethylene (HDPE), and linear low-density polyethylene (LLDPE), each with its unique properties and applications. The choice of catalyst, polymerization conditions, and post-polymerization processing can significantly influence the final properties of the polyethylene product.
Biological Role of Ethylene in Plants
Beyond its industrial significance, ethylene plays a critical role in plant biology. It is a plant hormone that regulates various physiological processes, including fruit ripening, senescence (aging), and responses to stress and injury. Ethylene production is triggered by a range of factors, including mechanical stress, infection, and senescence, and it acts as a signaling molecule to coordinate plant responses. The regulation of ethylene biosynthesis and signaling pathways is complex and involves multiple genes and enzymes. Understanding the biological functions of ethylene in plants has practical implications for agriculture, particularly in the development of strategies to control fruit ripening and extend shelf life, as well as to improve plant resilience to environmental stresses.
What is the primary method of industrial ethylene production?
+The primary method of industrial ethylene production is through the steam cracking of hydrocarbons, such as ethane and propane.
What is the biological role of ethylene in plants?
+Ethylene acts as a plant hormone, regulating processes such as fruit ripening, senescence, and responses to stress and injury.
What are some common applications of ethylene beyond polymer production?
+Beyond its use in polymer production, ethylene is used as a refrigerant, an anesthetic, and in the production of ethylene oxide, which is used in the manufacture of antifreeze and other chemicals.
In conclusion, ethylene (C2H4) is a compound of immense importance in both industrial chemistry and plant biology. Its unique chemical properties make it a versatile building block for the synthesis of various polymers and chemicals, while its biological functions are crucial for plant development and response to environmental cues. As research into ethylene continues, new applications and insights into its role in chemistry and biology are likely to emerge, further highlighting the significance of this simple yet multifaceted molecule.