Water Molar Mass: Calculate Easily

When discussing the properties of water, one of the fundamental aspects to consider is its molar mass. The molar mass of a substance is the mass of one mole of that substance, and it is expressed in units of grams per mole (g/mol). Water, being a compound made up of hydrogen and oxygen, has a molar mass that can be calculated based on the atomic masses of its constituent elements. In this article, we will delve into the calculation of the molar mass of water, exploring the steps involved and the underlying principles that make this calculation straightforward.
Key Points
- The molar mass of water is calculated by summing the atomic masses of two hydrogen atoms and one oxygen atom.
- The atomic mass of hydrogen is approximately 1.00794 g/mol, and the atomic mass of oxygen is approximately 15.9994 g/mol.
- Understanding the molar mass of water is crucial for various chemical calculations and applications, including the preparation of solutions and the determination of chemical reactions' stoichiometry.
- The calculation of water's molar mass involves basic arithmetic operations, making it accessible to individuals with a foundational knowledge of chemistry.
- Knowledge of the molar mass of water can be applied in numerous fields, including chemistry, biology, and environmental science, highlighting its significance in scientific inquiry and practice.
Calculating the Molar Mass of Water
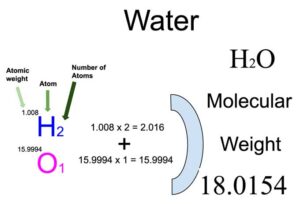
The chemical formula for water is H2O, indicating that one molecule of water consists of two hydrogen atoms and one oxygen atom. To calculate the molar mass of water, we need to know the atomic masses of hydrogen and oxygen. The atomic mass of an element is the average mass of its naturally occurring isotopes, and it is typically expressed in units of atomic mass units (amu) or grams per mole (g/mol).
The atomic mass of hydrogen (H) is approximately 1.00794 g/mol, and the atomic mass of oxygen (O) is approximately 15.9994 g/mol. Given this information, the molar mass of water (H2O) can be calculated as follows:
Molar mass of H2O = (2 * atomic mass of H) + atomic mass of O
Molar mass of H2O = (2 * 1.00794 g/mol) + 15.9994 g/mol
Molar mass of H2O = 2.01588 g/mol + 15.9994 g/mol
Molar mass of H2O = 18.01528 g/mol
Importance of Molar Mass in Chemistry
The molar mass of a substance, such as water, is a critical piece of information in chemistry. It serves as the basis for calculating the number of moles of a substance, which is essential for preparing solutions, calculating the quantities of reactants and products in chemical reactions, and determining the stoichiometry of reactions. Understanding the concept of molar mass and how to calculate it is fundamental to performing these tasks accurately.
| Element | Atomic Mass (g/mol) |
|---|---|
| Hydrogen (H) | 1.00794 |
| Oxygen (O) | 15.9994 |
| Water (H2O) | 18.01528 |

Applications of Molar Mass Calculations
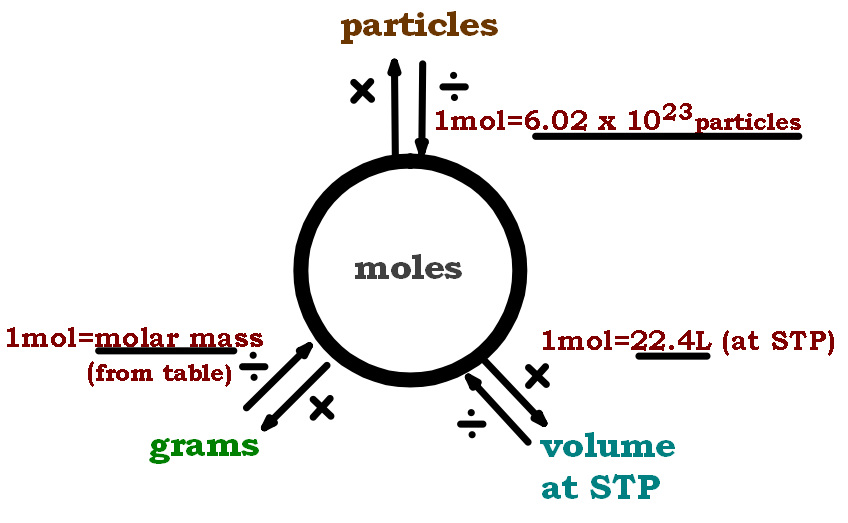
The ability to calculate the molar mass of substances, including water, has numerous applications across various fields. In laboratory settings, knowing the molar mass of substances is essential for preparing solutions of specific concentrations, a process that relies on the accurate measurement of masses and volumes. Additionally, in chemical engineering and industrial processes, understanding the molar masses of reactants and products is vital for optimizing reaction conditions, yields, and safety protocols.
Beyond the laboratory, the concept of molar mass and the ability to calculate it play significant roles in environmental science, biology, and medicine. For instance, in environmental science, calculating the molar masses of pollutants can help in assessing their impact on ecosystems and in developing strategies for their removal or mitigation. In biology and medicine, understanding the molar masses of biomolecules, such as proteins, nucleic acids, and drugs, is crucial for studying their functions, interactions, and therapeutic applications.
Conclusion and Future Perspectives
In conclusion, the calculation of the molar mass of water is a straightforward process that involves summing the atomic masses of its constituent atoms. This calculation not only demonstrates the fundamental principles of chemistry but also highlights the importance of understanding the composition and properties of substances at the molecular level. As science continues to evolve, the applications of molar mass calculations will expand, contributing to advancements in various fields and addressing complex challenges faced by society.
What is the molar mass of water, and how is it calculated?
+The molar mass of water is approximately 18.01528 g/mol, calculated by summing the atomic masses of two hydrogen atoms and one oxygen atom.
Why is understanding the molar mass of substances important in chemistry?
+Understanding the molar mass of substances is crucial for calculating the number of moles, preparing solutions, and determining the stoichiometry of chemical reactions, among other applications.
What are some practical applications of molar mass calculations?
+Molar mass calculations have applications in laboratory settings for solution preparation, in chemical engineering for process optimization, and in environmental science, biology, and medicine for understanding and manipulating biomolecules and pollutants.